WebPatient
The Sequence WebPatient Difference

Real-Time Eligibility Checks
Quickly determine a patient’s eligibility for a clinical study using real-time, logic-based algorithms that provide an instant indication of eligibility based on entered data.

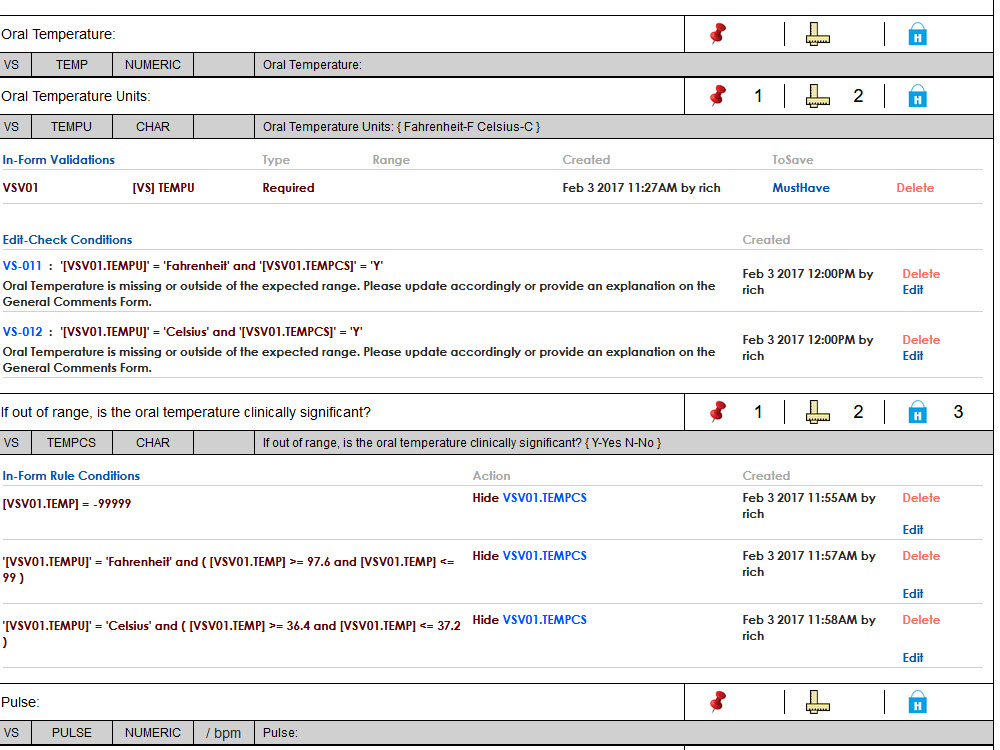
Configurable, Intuitive Forms
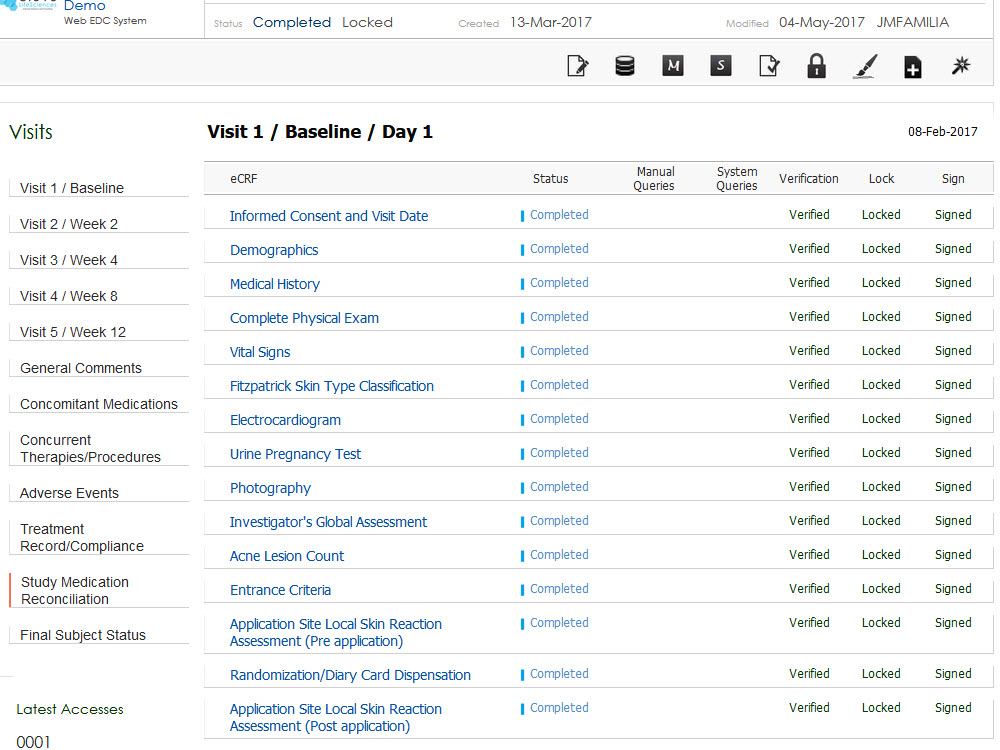
Leverage easy-to-use and highly configurable electronic case report forms (eCRFs) tailored to each protocol, reducing training time and improving data accuracy at the site level.

Centralized Review Workflows
Facilitate efficient communication between investigator sites, CROs, and sponsors with structured workflows for centralized eligibility reviews and approvals.

Automated Patient Data Distribution
Accelerate study progress with fast, automated sharing of patient data and eligibility statuses across the entire research team, ensuring all stakeholders are in sync.

Configurable Alerts via Email, Text, or Queries
Set up dynamic notifications that are triggered by any specific data point — or a combination of values — to keep research staff promptly informed of key events or data submissions.

Flexible Document Export for Study Closeout
Generate PDF archives of completed eCRFs, queries, and full audit trails in compliance with regulatory standards — ensuring a smooth and documented study closeout.

Task-Centric Oversight Tools
Stay ahead with interactive, role-specific dashboards and live reporting tools that help teams manage study progress, pending tasks, and enrollment status in real-time.
Additional Features
Support and Training
- Quick and efficient user training
- In-person training also available onsite at sponsor facilities
- Zero client requirements except browser
- Remote training available
- Technical support and dedicated 800 number per study
- Many levels of help desk and support options available
Security
- Role and workflow-based security
- Customizable event notifications
- Configurable workflow to fit customer’s organization
- Configurable down to the field level
- Configurable actions by role or user
Data / Hosting
- All data will be hosted on a validated, secure server located in a hardened, redundant hosting facility
- Full business continuity and disaster recovery functions provided
- All data will be backed up on a nightly basis both locally and to a different geographic location for redundancy
- 24/7/365 Performance and fault monitoring
- All files will be the client’s property and transferred upon request
Features
- AI Powered with Advanced Learning Algorithms
- Easy to use, feature rich forms and functions for evaluating Patient Eligibility for a study
- On-The-Spot, algorithm based indication of eligibility
- CRO/Sponsor eligibility review workflows
- Fast, automated communication of patient data and eligibility status across the research team and all desired personnel
- Extensive query management system to eliminate email, manage communications, protect patient data, maintain audit trails and follow 21 CFR Part 11 guidelines
- Email/Text/Query notifications can be configured to trigger on any data point or combination of data points
- Flexible PDF archiving of eCRFs/audit trail for study closeout
- Interactive task-based dashboards and real-time reporting
Benefits
- Greatly improves recruiting efficiency
- Promotes faster study execution
- Documents eligibility decisions and communications
- Requires minimal training with user friendly GUI
- Highly configurable, standards based
- Can be integrated with most EDCs or used as a stand-alone coding solution
- CDISC / CDASH standards
- 21 CRF Part 11 Compliant
